As the COVID-19 vaccines become more widely available, many people with a history of allergic reactions will need to determine their level of risk in receiving these two vaccines and any new ones that may become available.
From an article in The Journal of Allergy and Clinical Immunology: In Practice (December 30, 2020) this flow chart is useful for healthcare professionals and patients to determine who is at risk for a reaction and how to proceed:
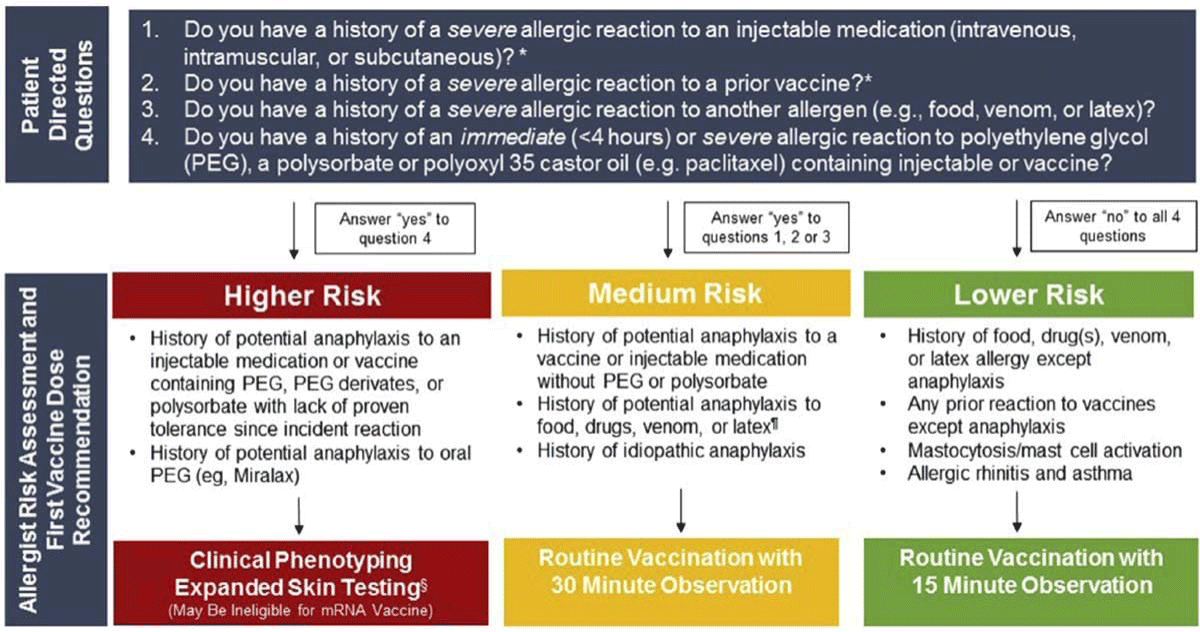
Patients should answer the following questions:
- Do you have a history of a severe allergic reaction to an injectable medication (intravenous, intramuscular, or subcutaneous)?
- Do you have a history of severe allergic reaction to a prior vaccine?
- Do you have a history of a severe allergic reaction to another allergen (e.g., food, venom, or latex)?
- Do you have a history of an immediate (less than 4 hours) or severe allergic reaction to polyethylene glycol (PEG), a polysorbate or polyoxyl 35 castor oil (e.g., paclitaxel) containing injectable or vaccine?
If you answer ‘No’ to all of the above, you are considered Lower Risk and should proceed with the vaccination with a 15-minute observation period after your injection.
If you answer ‘Yes’ to questions 1, 2, OR 3, you are at Medium Risk. Most people in this category can safely receive the vaccines, observing the 30-minute wait period in a medical setting. However, if you have specific questions or concerns please schedule an in-office or telehealth conference with one of the board-certified allergists at Atlanta Allergy & Asthma. Your doctor will be able to provide guidance on your risk factors and how to proceed.
If you answer ‘Yes’ to question 4, you are at Higher Risk. You should not receive either vaccine unless you are advised by an allergy expert. Additional testing may be required in determining your level of risk. (See below.)
What if You Have a Reaction to the First Dose?
The Pfizer/BioNTech and Moderna vaccines are administered in two doses. If you experience an allergic reaction to the first dose of the vaccine you may be ineligible for the second dose. The following flow chart from “JACI: In Practice” shows how healthcare professionals determine if a person should receive a second dose.
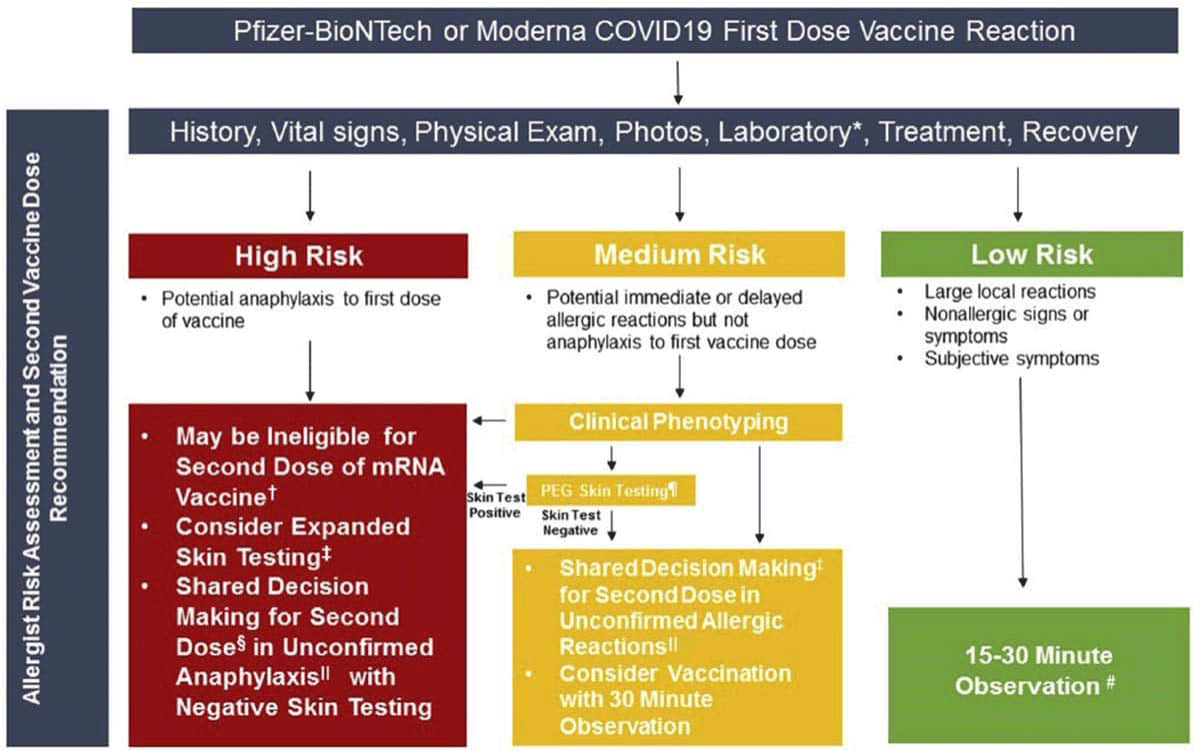
People who experience a possible allergic reaction (hives, swelling away from the injection site, generalized itching, flushing, throat swelling, chest tightness, shortness of breath, trouble breathing, faintness, low blood pressure, or loss of consciousness) after their first dose should consult with an allergy expert prior to receiving a second dose. After consultation, your allergist may recommend skin testing to help determine whether you can safely receive the second dose.